This week I want to show three
reversible color changing reaction, and they are blue bottle, red bottle,
and traffic light reaction. They are often used on magic shows or to perform demonstration
experiments. However, though it seems to be complex and the ingredient should
be the magician’s secret, I will reveal the secret to you today.
In fact, the mechanisms behind
these amazing experiments are similar and easy to understand. When the bottle is
shaken vigorously, one species in the solution would be oxidized (氧化) by the oxygen dissolving into the
solution, and the color would change due to the different color that oxidized
species has form the original one. After we put it aside and wait for a while,
we could observe the color gradually return to the initial one. That is because
the oxidized species has been reduced by the reductant (還原劑), usually glucose, in the
solution.
Here are some videos:
blue bottle
red bottle and blue bottle (they are combined to make purple bottle)
traffic light
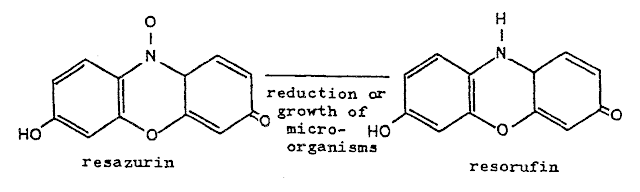
In the blue bottle reaction,
the species being oxidized is methylene blue (亞甲藍); in the read bottle reaction, that would be resazurin (刃天青); in the traffic light
reaction, that would be indigo carmine (靛胭脂). The indigo carmine has three oxidation states with
different colors so the color depends on which level it has been oxidized.
methylene blue:
resazurin:
colorless red
indigo carmine:
Reference:
1.http://ncsu.edu/project/chemistrydemos/Kinetics/Blue%20Bottle.pdf
2.https://en.wikipedia.org/wiki/Blue_bottle_(chemical_reaction)
3.http://case.ntu.edu.tw/magichem/blog/wp-content/uploads/2010/09/NTUCASEChemdemo_example.pdf


Wow~Amazing!! I've never thought that those species you mentioned can act like that!!
回覆刪除I wish I can play with it in the lab someday xdd.
回覆刪除That's incredible!
回覆刪除I'm a bit confused by the English name of the reactants haha .
It is a fun part to see color changes in chemical reaction, and it is MORE fun to see the color reverses!
回覆刪除作者已經移除這則留言。
回覆刪除